Dye-sensitized photosynthesis cell for oxidation of glycerol
24 March 2022
Dye-sensitized photoelectrochemical cells (DSPECs) are an extension of dye-sensitized solar cells (DSSCs) that are capable of producing electrical energy under irradiation with sunlight. By introducing a catalyst, a system can be developed that is capable of catalytic chemical conversions, producing fuels or chemicals instead of electricity.
A basic DSPEC operation involves water splitting, resulting in the generation of hydrogen as a main product and oxygen as a byproduct. The oxygen evolution reaction hampers the overall efficiency because water oxidation is a four-electron process. Therefore, there is an interest in exploring alternative oxidative reactions in DSPECs that preferably involve two-electron processes. Such anodic co-valorization not only enhances the efficiency of hydrogen generation, but can also produce commercially valuable compounds and thus extend the business case.
Synthesis of glyceraldehyde
In their paper in Angewandte Chemie, Reek and coworkers now present for the first time a DSPEC capable of oxidation of glycerol, an archetypical biobased compound found in animal and vegetable fats. They demonstrate its conversion into glyceraldehyde which finds application in, for instance, cosmetics and the preparation of polyesters and adhesives.
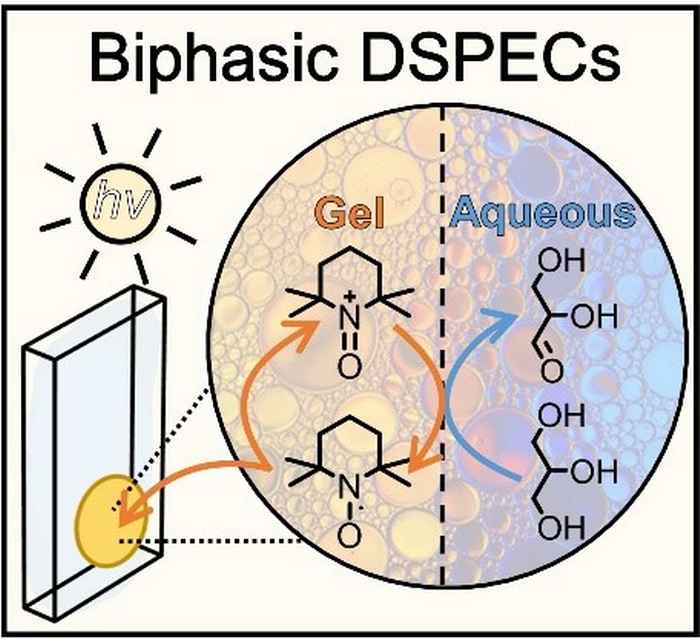
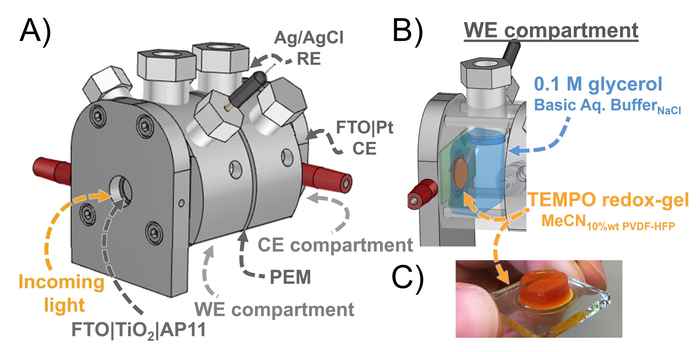
The researchers use a DSPEC fitted with a photoanode that is protected by an active gel layer that shuttles electrons between the photoexcited dye and glycerol at the biphasic interface. It thus results in a tenfold increase of the photocurrent density and product formation compared to an aqueous DSPEC system. The improved efficiency and high stability of the redox-gel-based DSPEC opens up the possibility for the photoconversion of other solely water-soluble including biomass-derived substrates such as cellulose. Furthermore, the concept of employing a redox-gel to enhance the stability of aqueous DSPECs can also be considered in the context of water-splitting DSPECs.
Abstract
This work reports an aqueous dye-sensitized photoelectrochemical cell (DSPECs) capable of oxidizing glycerol (an archetypical biobased compound) coupled with H2 production. We employed a mesoporous TiO2 photoanode sensitized with the high potential thienopyrroledione-based dye AP11, encased in an acetonitrile-based redox-gel that protects the photoanode from degradation by aqueous electrolytes. The use of the gel creates a biphasic system with an interface at organic (gel) electrode and aqueous anolyte. Embedded in the acetonitrile gel is 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), acting as both a redox-mediator and a catalyst for oxidative transformations. Upon oxidation of TEMPO by the photoexcited dye, the in situ generated TEMPO+ shuttles through the gel to the acetonitrile–aqueous interface, where it acts as an oxidant for the selective conversion of glycerol to glyceraldehyde. The introduction of the redox-gel layer affords a 10-fold increase in the conversion of glycerol compared to the purely aqueous system. Our redox-gel protected photoanode yielded stable photocurrent over 48 hours of continuous operation, demonstrating that this DSPEC is compatible with alkaline aqueous reactions.
Publication details
Didjay F. Bruggeman, Annechien A. H. Laporte, Remko J. Detz, Simon Mathew and Joost N. H. Reek: Aqueous Biphasic Dye-sensitized Photosynthesis Cells for TEMPO-based Oxidation of Glycerol Angewandte Chemie International Edition, First published: 10 March 2022. DOI: 10.1002/anie.202200175
See also
- Interview with Joost Reek in UvA magazine Folia (in Dutch)
- Research group Homogeneous, Supramolecular and Bio-Inspired Catalysis