Novel method for direct synthesis of meta-arylated anilines
16 January 2024
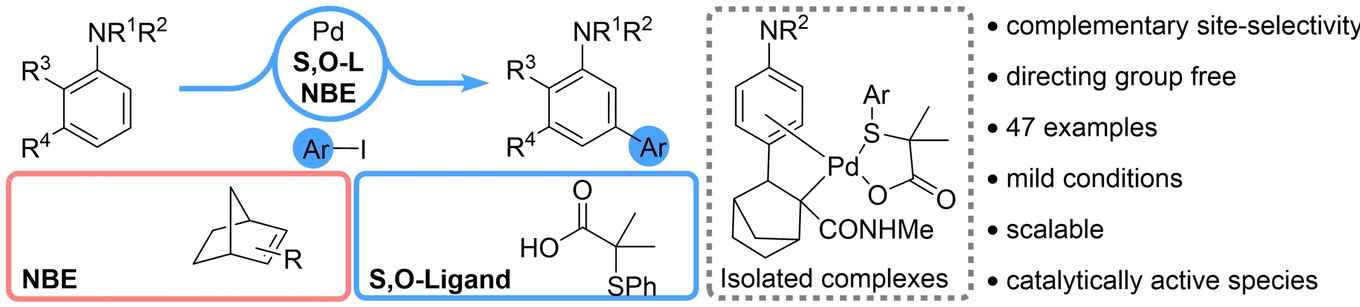
The novel method provides an invaluable synthetic tool for late-stage diversification of biological active compounds and offers access to potentially new molecules with improved properties. By the judicious choice of the S,O-ligand and norborene mediator, more challenging substrates including ortho-substituted anilines and aryl iodides lacking an electron withdrawing group at the ortho-position are also tolerated.
The Angewandte Chemie paper provides some useful insights into the factors influencing the meta-C−H activation step. It is suggested that the meta-substituent on the aniline substrate as well as the amide group and the bridgehead substituent in the norborene mediator have a positive effect in promoting the meta-C−H activation.
The research was carried out in cooperation with Prof. Bas de Bruin of the research group Homogeneous, Supramolecular and Bio-inspired catalysis, and was supported by the Dutch Research Council NWO as part of its ECHO funding scheme.
Abstract of the paper
Aromatic amines are ubiquitous moieties in organic molecules and their direct functionalization is of great interest in many research areas due to their prevalence in pharmaceuticals and organic electronics. While several synthetic tools exist for the ortho- and para-functionalization of anilines, the functionalization of the less reactive meta-position is not easy to achieve with current methods.
To date, the meta-C−H arylation of aniline derivatives has been restricted to either the use of directing groups & templates, or their transformation into anilides & quaternary anilinium salts. Herein, we report the first general and efficient meta-C−H-arylation of non-directed aniline derivatives via cooperative catalysis with a palladium–S,O-ligand–norbornene system. The reaction proceeds under mild conditions with a wide range of aniline derivatives and aryl iodides, while being operationally simple and scalable. Our preliminary mechanistic investigation–including the isolation of several palladium complexes and deuterium experiments–reveal useful insights into the substituent-effects of both the aniline-substrate and the norbornene-mediator during the meta-C−H activation step.
Paper details
Verena Sukowski, Manuela van Borselen, Simon Mathew, Bas de Bruin, and M. Ángeles Fernández-Ibáñez: meta-C−H Arylation of Aniline Derivatives via Palladium/S,O-Ligand/Norbornene Cooperative Catalysis. Angew. Chem. Int. Ed. 2023, e202317741 DOI: 10.1002/anie.202317741