Novel isocyanate-free synthesis of polyurea
9 November 2022
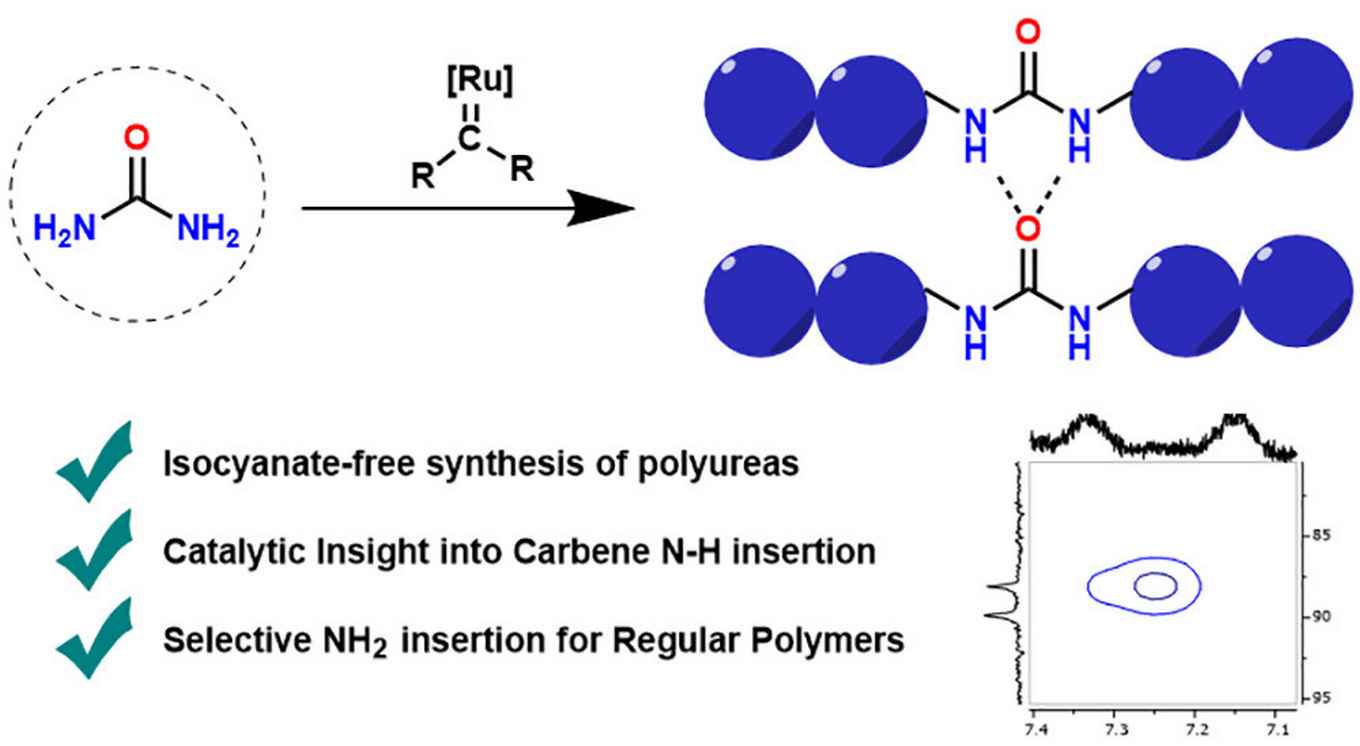
Polyurea-based elastomers display unique material properties such as high tensile strength and tear resistance. This has led to their widespread use in products such as foams and coatings. However, common polyurea synthesis is based on the use of isocyanates. This is problematic because of their toxicity, reactivity and sensitivity to humidity, so that there is much interest in the use of alternative, more benign monomers. The paper in Macromolecules, authored by Felix de Zwart, a PhD candidate with Prof. Bas de Bruin, describes the synthesis of polyurea through ruthenium catalyzed carbene insertion into the N–H bonds of urea. This led to polymers with material properties that are tuneable through side-chain or main-chain substitution. In general, this proof-of-concept paper shows the possibility of using diazo compounds in combination with transition-metal catalysis to furnish novel routes toward isocyanate-free polyureas.
Abstract of the paper
Polyureas have widespread applications due to their unique material properties. Because of the toxicity of isocyanates, sustainable isocyanate-free routes to prepare polyureas are a field of active research. Current routes to isocyanate-free polyureas focus on constructing the urea moiety in the final polymerizing step. In this study we present a new isocyanate-free method to produce polyureas by Ru-catalyzed carbene insertion into the N–H bonds of urea itself in combination with a series of bis-diazo compounds as carbene precursors. The mechanism was investigated by kinetics and DFT studies, revealing the rate-determining step to be nucleophilic attack on a Ru–carbene moiety by urea. This study paves the way to use transition-metal-catalyzed reactions in alternative routes to polyureas.
Paper details
Felix J. de Zwart, Petrus C. M. Laan, Nicole S. van Leeuwen, Eduard O. Bobylev, Erika R. Amstalden van Hove, Simon Mathew, Ning Yan, Jitte Flapper, Keimpe J. van den Berg, Joost N. H. Reek, and Bas de Bruin: Isocyanate-Free Polyurea Synthesis via Ru-Catalyzed Carbene Insertion into the N–H Bonds of Urea. Macromolecules 2022 Publication date October 17, 2022 DOI: 10.1021/acs.macromol.2c01457
See also
Website research group Homogeneous, Supramolecular and Bio-inspired Catalysis.