New method for constructing 8-membered ring compounds
Metalloradical catalysis provides efficient solution for medium-sized ring synthesis
24 April 2020
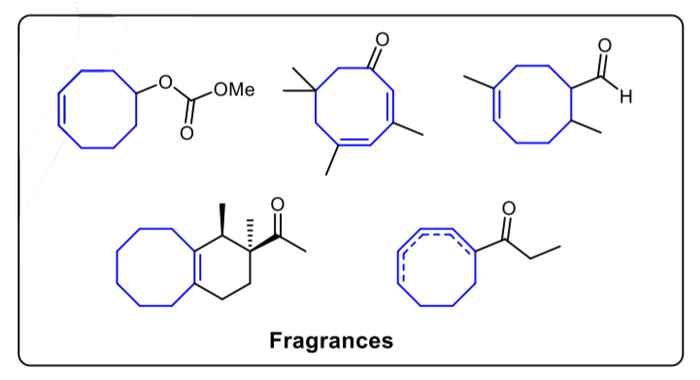
Medium-sized cyclic compounds, especially 8-membered rings, widely exist in many natural products, fuels, fragrances and catalysts. Although the importance of developing an efficient method to build these ring structures is well-known, synthesizing such compounds in the lab is still quite challenging. It requires harsh reaction conditions and is often accompanied by poor functional group tolerance, low yield and a great number of side products.
In their Angewandte Chemie paper, researchers of the HIMS research group Homogeneous, Supramolecular and Bio-Inspired Catalysis present a solution. Based on metalloradical catalysis developed by Prof. Bas de Bruin (Bio-inspired Sustainable Catalysis), they used a cobalt catalyst to enable the selective synthesis of medium-sized ring compounds. The novel catalytic reaction provides access to two types of novel 8-membered ring systems: dibenzocyclooctenes and monobenzocyclooctadienes. These structures are confirmed by many detection techniques (nuclear magnetic resonance, NMR; single crystal X-ray Diffraction, SC-XRD; high-resolution mass spectrometry, HRMS) and the mechanism is strongly supported by computational studies (density functional theory, DFT). The reaction has excellent chemoselectivity in the construction of medium-sized ring structures under mild reaction conditions and enables the synthesis of a variety of novel 8-membered ring compounds from a broad substrate scope. The expected and usually favoured 6-membered rings are not formed at all. The cobalt complex ([CoII(TPP)], TPP = tetraphenylporphyrin) used as a catalyst in this reaction is commercially available, and relatively inexpensive compared to noble metal catalysts.
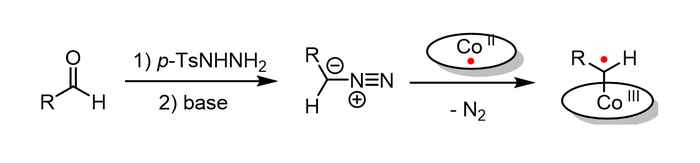
Two different ring-closure mechanisms
In this reaction, cobalt(II) complexes react with carbene precursors to produce cobalt(III)-carbene radical intermediates (image above), which enable unusual catalytic radical-type pathways. The carbene precursor can be generated from readily available aldehyde via an in situ generation of the N-tosylhydrazones and diazo compound by a non-catalytic process. It is subsequently trapped by the cobalt(II) complex, which significantly simplifies the synthetic methodology and makes the metalloradical strategy widely applicable.
Based on computational studies, it was established that the reactions proceed via hydrogen atom transfer (HAT) from the bis-allylic/benzallylic C-H bond to the carbene radical. This is followed by two divergent processes for ring-closure to arrive at two different types of 8-membered ring products. While the dibenzocyclooctenes are formed by dissociation of o-quinodimethanes (o-QDMs) which undergo a re-aromatizing 8π-cyclization, the ring-closure to the monobenzocyclooctadienes involves a radical-rebound step, in which the terminal carbon atom of the allyl radical moiety attacks the cobalt(III)-carbon bond. The latter leads to simultaneous new C-C bond formation and homolysis of the weak Co-C bond to form the final product.
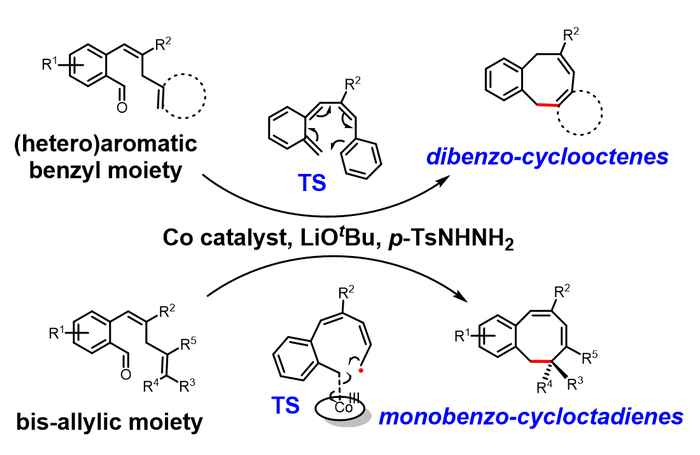
The radical-rebound mechanism of monobenzocyclooctadienes is also supported by experimental results. By using asymmetric cobalt porphyrin catalyst, chiral 8-membered ring products can be obtained, which strongly suggests that the ring-closure proceeds via radical-rebound pathway. It is also the first example of catalytic enantioselective 8-membered ring synthesis via a carbene radical intermediate.
Publication details
Minghui Zhou, Marianne Lankelma, Jarl Ivar van der Vlugt, Bas de Bruin: Catalytic Synthesis of 8-Membered Ring Compounds via Cobalt(III)-Carbene Radicals. Angew. Chem. Int. Ed. 2020, 142, 552–563 DOI: 10.1002/anie.202002674