Supramolecular substrate organization makes for a selective H borylation catalyst
16 July 2019
Sustainable synthesis protocols are of crucial importance for the development of waste-free chemical processes that produce the materials our society needs. In recent years, the CH activation reaction has attracted a lot of attention as it avoids the use of waste-producing activation reactions (such as bromination) and enables the introduction of functional groups in chemical structures that already contain many functionalities. To make this key reaction protocol industrially relevant, however, it is paramount to develop selective catalysts that can target specific CH bonds, as most compounds contain multiple CH bonds.
In the past decades, chemists have developed many catalysts for CH activation based on various transition metals (mostly Ru, Ir, Pd). In particular, iridium catalyzed borylation is an interesting reaction. The borylated product allows multiple follow-up reactions that can further selectively functionalize the compound with a variety of groups. However, although for many substrates selective protocols have been developed, ortho-selective CH borylation for amide containing substrates has so far not been possible as this concerns a deactivated CH bond.
Substrate organization
In order to develop CH activation protocols for the important class of amide containing aromatic rings, the Amsterdam researchers at the group for Homogeneous, Supramolecular and Bio-inspired catalysis led by Prof. Joost Reek have now applied their concept of substrate orientation to this particular problem. This concept was inspired by the working principles of natural enzymes that bind molecules in well-defined pockets close to their active sites and thereby use pre-organisation as a strategy to induce selectivity. PhD student Shaotao Bai succeeded in mimicking this enzymatic behaviour with an iridium catalyst that is functionalized with an indole amide binding site. It properly organizes the amide substrate for ortho selective CH activation.
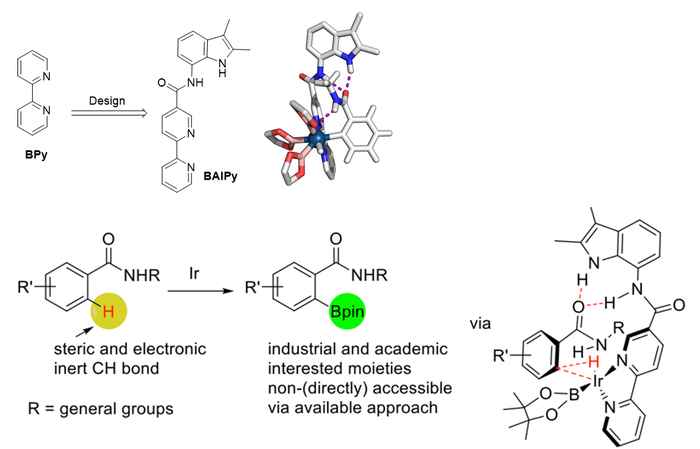
In their publication, which has just been accepted by Angewandte Chemie International Edition, the researchers present their novel supramolecular iridium catalyst. By combining experimental data and DFT calculation, they were able to elucidate the reaction mechanism and demonstrate that the hydrogen bonds are important for the pre-organization and activation. Substrate organization takes place via the formation of three hydrogen bonds between the substrate and the catalyst. If not all three hydrogen bond can be formed, the selectivity for the reaction drops substantially.
The research shows that the catalyst is of broad use: 26 different substrates have been selectively borylated. The catalyst is easy to prepare and therefore accessible on a larger scale, and the reaction has been performed at the multigram scale. Thus, after earlier research has demonstrated that substrate orientation is a useful tool in hydroformylation and hydrogenation, this work now shows that it also a powerful concept in the regioselective CH borylation.
The research is part of UvA's research priority area Sustainable Chemistry and was funded by Joost Reek's ERC Advanced Grant in 'Nature Inspired Transition Metal Catalysis' (CBB) and CSC fellowship (SB).
Publication details
Shaotao Bai, Charles Bheeter and Joost N. H. Reek: Hyrdogen bond directed ortho-selective C-H borylation of secondary aromatic amides . Angewandte Chemie International Edition, accepted. DOI:10.1002/anie.201907366
Website Reek research group: Homogeneous, Supramolecular and Bio-inspired catalysis.