Efficient alternative for multistep synthesis of aniline-based drug precursors
18 April 2019
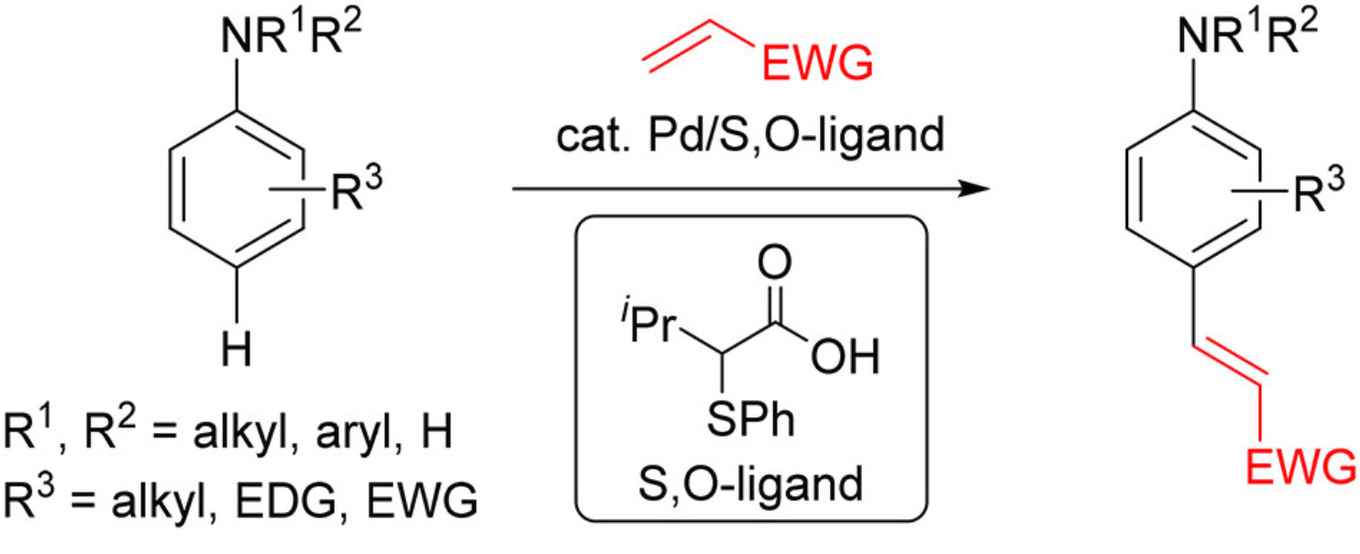
Aromatic amines are ubiquitous structural motifs in natural products, pharmaceuticals, fluorescent dyes, and organic functional materials. In particular, para-olefinated anilines are useful building blocks for the synthesis of diverse natural products or pharmaceutical drugs such as Rilpivirine. Nevertheless, to access this type of molecules, a multistep synthesis is necessary, increasing the cost of the process and the waste formation.
Direct functionalization of C-H bonds
A more efficient alternative to this multistep synthesis is the direct functionalization of C-H bonds. However, selective C–H functionalization reactions of aniline derivatives at remote positions are rare. In particular, a general strategy for para-selective C–H olefination of aromatic amines is still elusive.
Now, Fernández Ibáñez's PhD student Kananat Naksomboon has devised a general method for para-selective C–H olefination of aniline derivatives by applying a new catalytic system based on Pd/S,O-ligand catalysis. She cooperated with Jordi Poater of the University of Barcelona and Matthias Bickelhaupt of Vrije Universiteit Amsterdam.
Mild conditions and good yields
The reaction described in JACS proceeds under mild reaction conditions with a broad range of anilines, including mono-, di-, and trisubstituted anilines bearing electron-donating and -withdrawing groups. In total, 42 aniline derivatives underwent para-selective C–H olefination in good yields using the developed methodology.
The research also shows that it is possible to use oxygen as the only oxidant and that this methodology is operationally simple and scalable. The S,O-ligand, which was recently discovered at Fernández Ibáñez’s research group, is responsible for the dramatic improvements in substrate scope and the high para-selectivity observed in this transformation. Preliminary mechanistic studies suggest that the ligand promotes the C–H bond cleavage, which is the rate-limiting step.
Further applications and mechanistic studies are currently ongoing in the HIMS Synthetic Organic Chemistry laboratory.
Publication details
Kananat Naksomboon, Jordi Poater, F. Matthias Bickelhaupt, and M. Ángeles Fernández-Ibáñez: para-Selective C–H Olefination of Aniline Derivatives via Pd/S,O-Ligand Catalysis. J. Am. Chem. Soc., Article ASAP DOI: 10.1021/jacs.9b01908