UvA chemists shed light on intermediate steps in cascade reactions
19 March 2018
Many complex catalytic systems involve tandem reactions where two or more catalytic cycles are combined into one synthetic operation. Designing tandem catalysts is challenging, since they must include a transition step between the two catalytic sites.
If the two catalytic cycles are separate (for example an acid-catalysed reaction at one site, followed by a base-catalysed reaction at another), they can be treated separately. But when a catalytic cycle requires both sites, the transition step between them cannot be ignored. The problem is that this step typically involves short-lived intermediates that diffuse across the catalyst surface, and therefore is extremely difficult to follow.
Now, Thierry Slot, a PhD student at HIMS presents a theoretical concept that describes tandem reactions for active particles on active surfaces. The research, supervised by Gadi Rothenberg of the Heterogeneous Catalysis and Sustainable Chemistry research group, is part of the NWO TOP-PUNT project Catalysis in Confined Spaces.
Active doughnut
Based on a simple pen-and-paper analysis, the researchers show that the adsorption and diffusion steps in catalytic tandem systems are governed by a set of rules relating to the size of the catalytic particles and their interactions with short-lived reaction intermediates. These rules can account for adsorption, diffusion and reaction kinetics in a variety of experimental cases.
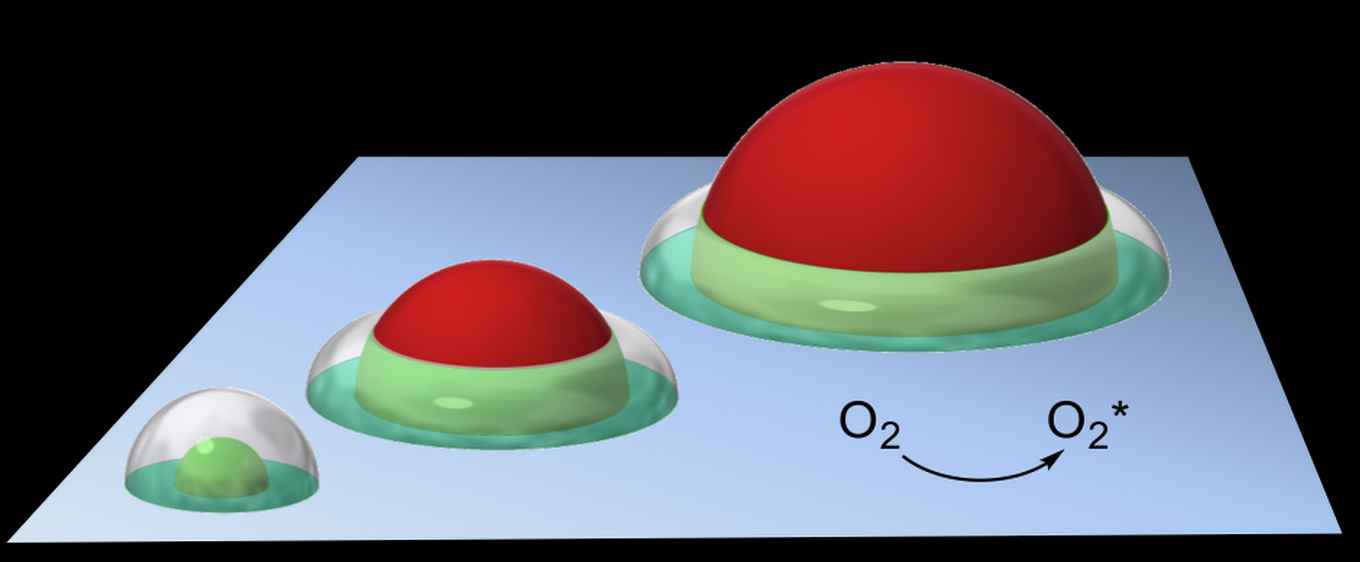
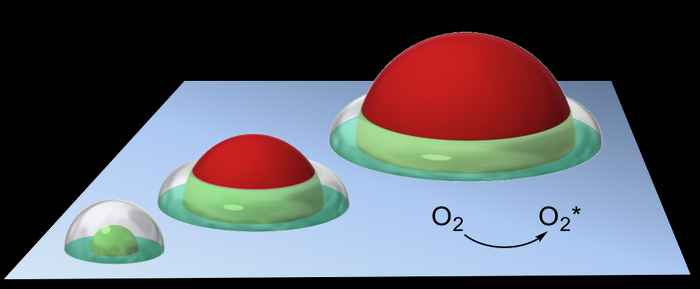
Central to the analysis is the concept of the so‐called 'active doughnut' an intuitive model developed earlier by Rothenberg’s group for selective oxidation reactions, defining the active catalytic region around the supported particles (see figure below).
Correctly reporting turnover numbers
This new theoretical concept has two important practical implications. First, it shows that the tandem reaction rate is not proportional to the number of active sites, but rather to the number of 'communicative' active sites—those available to the reaction intermediates during their respective lifetimes. If a catalytic particle is too large, for example, much of its surface area will go unused. Furthermore, if small particles are bunched together, their active volumes overlap, and the catalytic surface is under-utilized.
This has important implications for reporting turnover numbers (TONs), whose values are underestimated when determined in either of these situations. Practically speaking, the most efficient particle-surface tandem catalysts are those where both particle size and inter-particle distance are of the same order of magnitude as the active doughnut thickness.
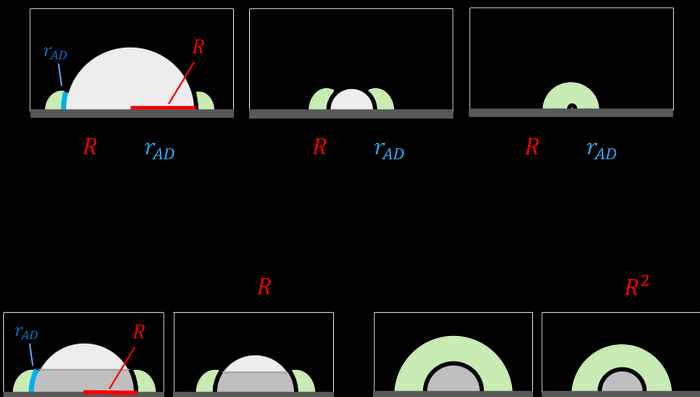
Effect of particle size
Second, the concept generates an important testable prediction concerning the dependence of the reaction rate on the particle size. In most catalytic systems, the total surface area of particles is much smaller than the surface area of the support. This turns particle surface area into a limiting factor in tandem catalysis. If the particle is small, intermediates can diffuse to its entire surface, and the rate of the reaction will have a quadratic relation to the particle radius. In contrast, if particles are too large, only a belt-shaped region surrounding the particle base participates in catalysis, and the reaction rate becomes linearly dependent on particle radius.
By synthesising and testing supported catalyst particles of different sizes, you will reach a point where the dependence of the rate on the particle size will shift from quadratic to linear. This point can be determined experimentally, giving important mechanistic information on the transition step between the two sites, and ultimately helping in the design of better cascade catalysts.
Open-access article
Cooperative surface-particle catalysis: the role of the “active doughnut” in catalytic oxidation. T.K. Slot, D. Eisenberg and G. Rothenberg, ChemCatChem, 2018, EarlyView. DOI: 10.1002/cctc.201701819 (open access)