Taming radicals to synthesize napthalenes and dienes
23 October 2017
Both types of molecules are of interest to the chemical industry. Dihydronaphthalenes are used in the production of pesticides and as precursors in drug molecule synthesis. Dienes are relevant, amongst others, for polymerizations (to yield plastics) and epoxidations (to produce chemical intermediates).
Bio-inspired catalyst
In chemistry, radical species (containing an unpaired electron) are usually considered too reactive to be selective, thus resulting in a plethora of reaction products. At the Homogeneous, Supramolecular and Bio-Inspired Catalysis (HomKat) Group of HIMS, however, Bas de Bruin and co-workers have developed an approach to tame radicals by employing a bio-inspired, heme-like cobalt catalyst and taking advantage of the intrinsic reactivity of the cobalt(III)–carbene radical intermediate. The catalyst is cheaper and more available than catalysts used in currently available synthesis methods. Furthermore, it gives access to new products.
One-pot synthesis
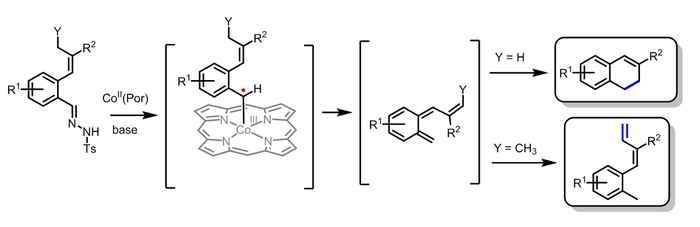
The method reported by the Amsterdam researchers for dihydronapthalene synthesis is far less difficult and elaborate than current methods. The developed catalytic one-pot route, employing metalloradical catalysis, results in a cheaper and more efficient process and provides alternative synthetic possibilities using different starting materials. Furthermore the mechanism of the reaction is highly interesting, as the reaction proceeds via highly unusual and unexpected intermediates.
PhD student Colet te Grotenhuis used the metalloradical approach to synthesize the dihydronaphthalenes, which contain two fused rings, starting from compounds containing only one of those rings. Assisted by chemistry master's students she investigated the effect of variations in the substrate. Various R1 functionalities are well tolerated, but unexpectedly variation of the R2 group proved to have a surprisingly big influence on the obtained yields. The unusual mechanism of the reaction explains this behavior. DFT calculations and radical trapping experiments pointed to the formation of unexpected and unusual carbene radical and o‑quinodimethide intermediates. The latter ring-close to the desired double-ring products. This explains why the vinyl R2 group has such a large influence.
Dienes
The work was continued by changing the properties of the allylic position Y, since this was again expected to have a large influence on the ring-closing step. Remarkably, using substrates with an alkyl group at Y resulted in formation of dienes instead of dihydronaphthalenes. This not only provided direct proof for the formation of reactive, non-aromatic o-quinodimethide intermediates, but also provides an interesting and selective route for the synthesis of a wide variety of aryl-functionalized dienes. DFT calculations confirmed this and gave additional insights into the unusual mechanism proceeding via controlled radicals and o-quinodimethides.
Article
Colet te Grotenhuis, Braja G. Das, Petrus F. Kuijpers, Wouter Hageman, Mees Trouwborst and Bas de Bruin: Catalytic 1,2-dihydronaphthalene and E-aryl-diene synthesis via CoIII–Carbene radical and oquinodimethane intermediates. Chemical Science, published 12 October 2017. DOI: 10.1039/c7sc03909c