X-ray spectroscopy reveals details of capacity fading in Li-ion batteries
First study of electrodes under operating conditions
2 December 2016

The research, performed in collaboration with the Technical Electrochemistry research group of Prof. Dr Hubert Gasteiger at the Technical University of Munich (Department of Chemistry), has recently been published in Journal of Materials Chemistry A, a high impact journal on materials for energy and sustainability.
Capacity fading
Li-ion batteries are widely used in applications such as mobile phones and laptops. They also hold the key to future electromobility as they have the potential to substantially increase the driving range of electric vehicles. This does however require an increase in their energy density by at least a factor of 2.5. To meet this goal a series of high energy density cathode materials have been developed. Among these manganese-oxide based materials such as LiNiMnCoO (NMC) seem especially promising.

Unfortunately Li-ion batteries containing these new cathode materials show severe capacity fading. Manganese, nickel and cobalt ions dissolve from the cathode and diffuse towards the anode where they cause irreversible side reactions that hamper optimal battery performance. In particular the manganese accumulation on the anode has a detrimental effect.
A detailed mechanistic understanding of the anode side reactions is crucial to reduce or prevent the unwanted side effects and thus optimize battery performance. Key to this all is the determination of the oxidation state of manganese, which determines its reactivity. In recent years this has been the focus of many research projects, alas with contradictory results.
Operando spectroscopy
The Amsterdam and Munich researchers now deliver a major contribution to this field by performing, for the first time, X-ray spectroscopy under operating conditions. Their results unequivocally show that the migrated manganese at the anode has an oxidation state of +2. The same holds for nickel and cobalt.
This result contradicts many previous observations in which various oxidation states were reported ranging from 0 to +4. The researchers attribute these differences to the fact that all previous results were obtained through ex situ analysis where electrodes are investigated after recovery from a working battery cell. There the procedures of electrode harvesting and preparation significantly influence the observed oxidation state.
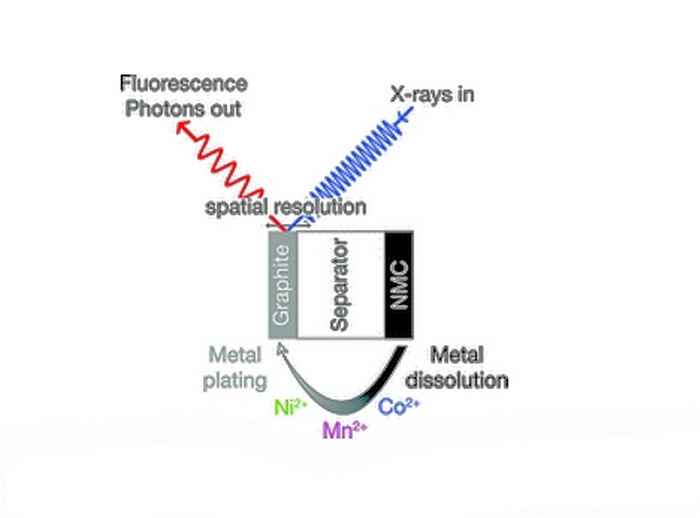
In contrast, the new technique of operando X-ray spectroscopy determines the oxidation states during battery operation. This provides a far more realistic view of the state of the electrode and excludes the influence of electrode preparation procedures such as washing and drying. The research was performed at the SAMBA beamline of the Soleil synchrotron in Saint-Aubin, France, using dedicated X-ray absorption spectroscopy (XAS) electrochemical cells equipped with NMC cathodes and graphite anodes, as previously developed by Tromp.
Towards more stable battery systems
The operando experiments allowed both the time/voltage and spatially resolved determination of metal concentration and oxidation state of the manganese, nickel and cobalt deposits on the graphite electrode. This also established that NMC shows a strong increase of the metal dissolution rate if the upper cut off potential exceeds 4.6 V.
The research thus provides profound insight in the stability and deactivation of Li-ion batteries equipped with NMC cathodes, establishing the importance of dedicated operando characterization for a comprehensive understanding of the battery processes.
Aiming for more stable battery systems, further research now focusses on the use of different transition metal compositions for the electrode and on the application of electrolyte solvent additives to influence the transition metal dissolution behaviour.
The research described here is part of a bigger energy research theme in the group of Moniek Tromp at the UvA's research priority area Sustainable Chemistry, investigating different battery systems for both mobility as well as stationary storage applications, with differing requirements. Old and new battery systems are characterized in detail to obtain profound understanding of their cycling behaviour in order to increase their energy capacity as well as long term stability. Another example of the research can be found in this leaflet (PDF).
Article
Johannes Wandt, Anna Freiberg, Rowena Thomas, Yelena Gorlin, Armin Siebel, Roland Jung, Hubert A. Gasteiger, Moniek Tromp: Transition metal dissolution and deposition in Li-ion batteries investigated by operando x-ray absorption spectroscopy J. Mater. Chem. A, 2016, 4, 18300-18305 DOI: 10.1039/C6TA08865A