'Radical' approach yields catalyst for sustainable indene synthesis
Averting the use of noble metals through bio-inspired metallo-radical catalysis
6 July 2016
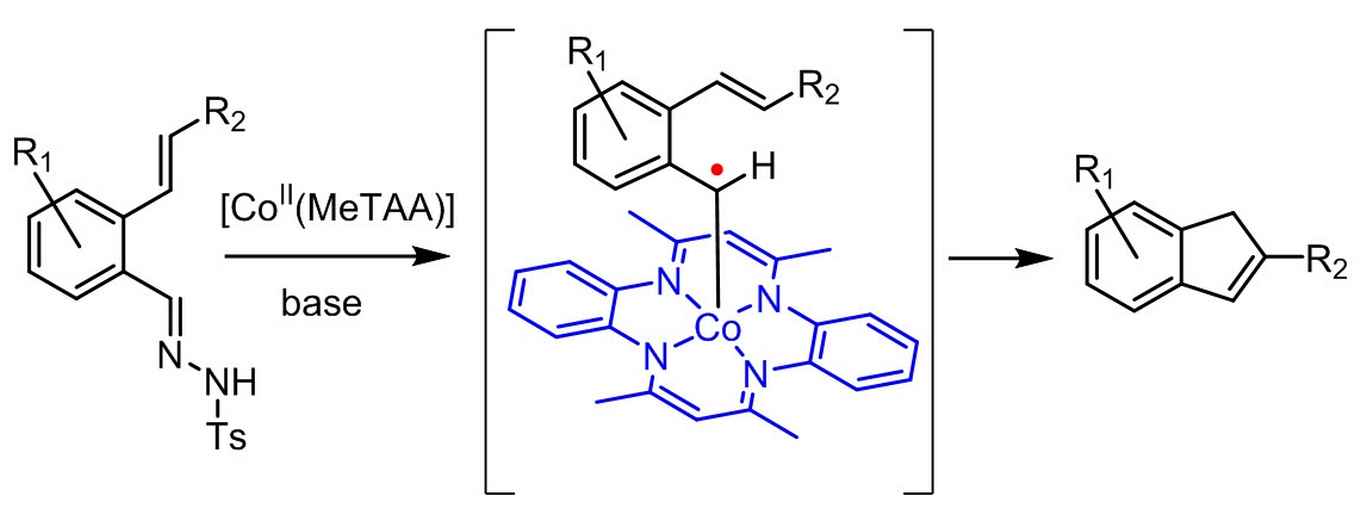
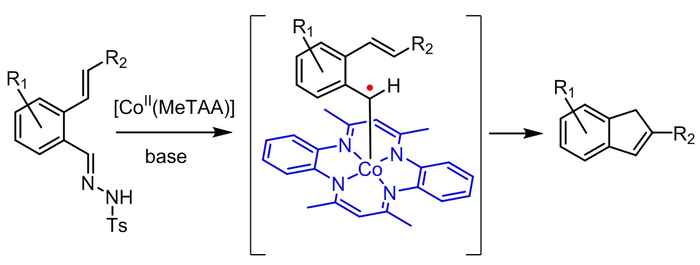
Indenes are valuable building blocks of a variety of natural products, pharmaceuticals and other bio-active compounds. They also have widespread applications in metal complexes for catalytic use in e.g. olefin polymerization. As such, there is a demand for the development of fast, efficient and broadly applicable methods for indene synthesis from readily available starting materials.
Bio-inspired approach
The strategy now reported by the UvA's SusChem team contributes to this development by providing a sustainable catalytic route to indene synthesis. It resulted from their new, bio-inspired approach in the field of so-called metallo-radical catalysis. Here the intrinsic radical-type reactivity of first row transition metals is utilized, where most currently applied catalytic approaches are aimed at preventing radical formation.
According to research leader Bas de Bruin the concept of metallo-radical catalysis enables chemists to move away from the current use of expensive noble metal catalysts and use cheap metals instead. Inspired by the performance of natural metallo-enzymes, De Bruin and his co-workers at the UvA's research priority area Sustainable Chemistry are currently developing more future catalysts also based on the concept of radical reactivity.
Publication
Das, B.G.; Chirila, A.; Tromp, M.; Reek, J.N.H; de Bruin, B.: CoIII-Carbene Radical Approach to Substituted 1H-Indenes J. Am. Chem. Soc. 2016 Published online June 24, 2016 DOI:10.1021/jacs.6b05434