Molecular filter is promising purifier for polyethylene production
Metal Organic Framework can be viable alternative for energy-demanding catalytic acetylene conversion
23 May 2016
Much of the world's plastic is made from ethylene gas, which is contaminated with acetylene gas. Removal of this contamination is essential because acetylene poisons the polymerization catalyst. The research now published in Science shows that filtering out acetylene using MOFs yields ethylene at the high purity that industry demands while sidestepping the current need to convert acetylene to ethylene via a costly, energy consuming catalytic process.
Porous crystals
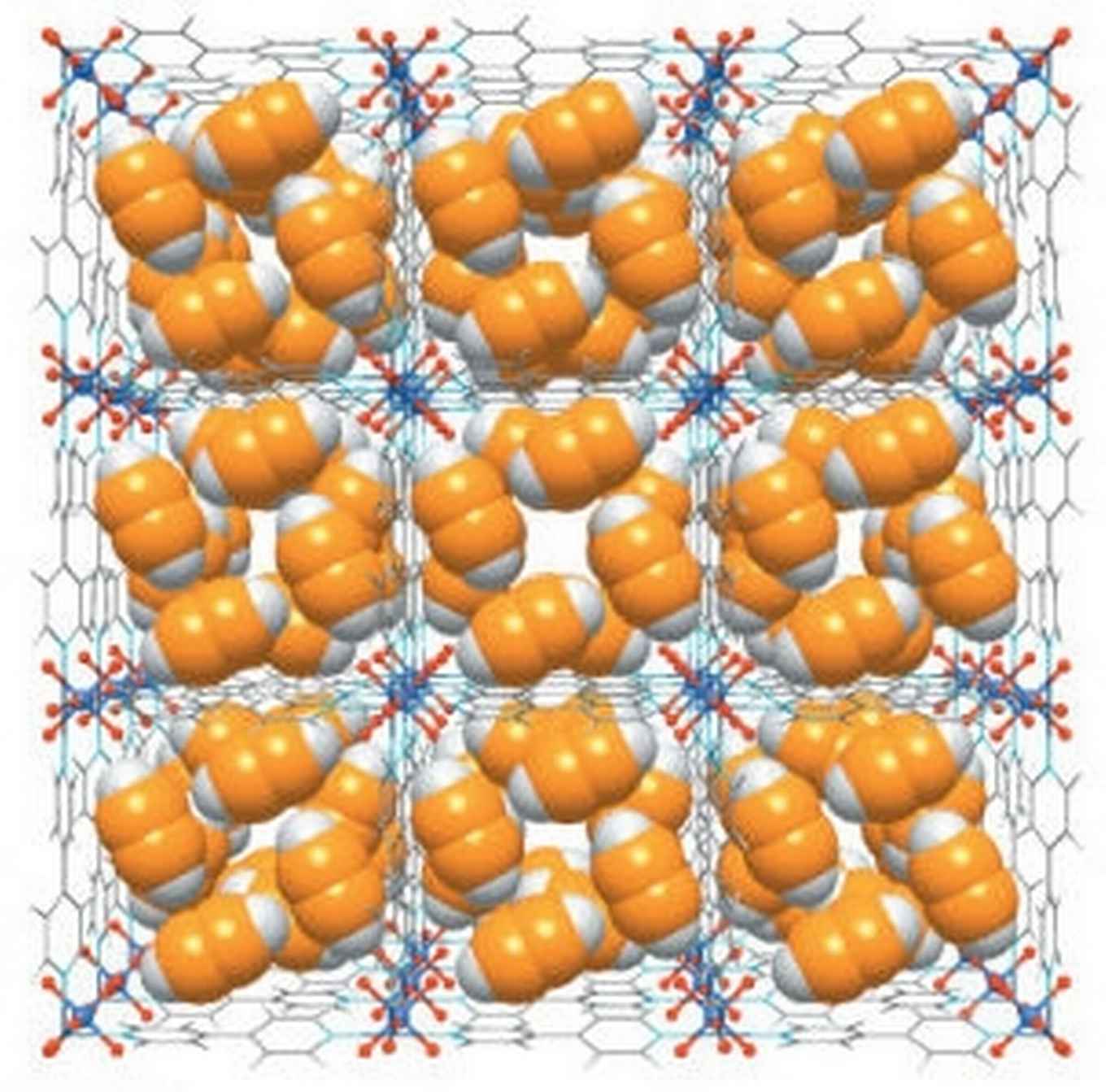
The research focused on a family of MOF materials called SIFSIX, discovered in the 1990s. MOFs are porous crystals that under a microscope look a bit like a building under construction - lots of girders with space in between. The SIFSIX group gets its name from some of its girders, which are formed from silicon (Si) and six atoms of fluorine (F6).
When ethylene is passed through these MOFs, the fluorine attracts and captures most of the acetylene contaminant, letting the now-purified ethylene to pass unhindered.
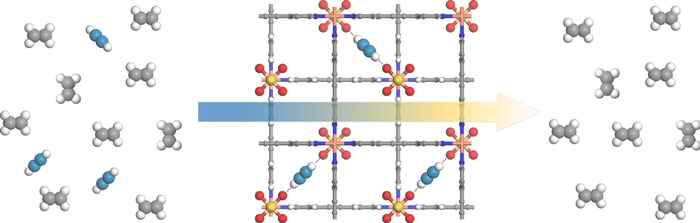
Varying the size of the pores allows the MOFs to filter ethylene-containing acetylene in concentrations of anywhere from 1 percent to 50 percent, which are typical in industry. As a result the amount of acetylene in ethylene can be lowered to less than 2 parts per million (ppm), which is lower than the 5 ppm that polyethylene manufacturing requires.
Record selectivity and adsorption capacity
Professor Krishna of the Computational Chemistry research group at HIMS contributed to the evaluation of the SIFSIX material with regard to their performance under industrially relevant conditions. Not only are the SIFSIX materials superior to competing MOFs, they also display significant benefits in comparison to other filtering materials.
As it turns out the SIFSIX MOFs set records among adsorbent materials for both selectivity (the ability to attract the acetylene only while allowing the ethylene to pass) and adsorption capacity. According to the research team, the results show that the SIFSIX group offers a viable alternative to standard industrial practice.
The MOFs were created and investigated in great detail by researchers based at China's Zhejiang University (by Huabin Xing), Ireland's University of Limerick, (Michael Zaworotko) and the University of Texas - San Antonio in the U.S. UT-San Antonio's Banglin Chen sensed the significance of SIFSIX MOFs for this application, and organized and led the team. Researchers of the National Institute of Standards and Technology (NIST) and Saudi Arabia's King Abdullah University of Science and Technology also contributed.
Article
X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M.J. Zaworotko and B. Chen: Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science, 19 May 2016. DOI:10.1126/science.aaf2458.