Novel porous materials enable difficult gas separations
HIMS researcher Rajamani Krishna contributes to international research projects
21 March 2016
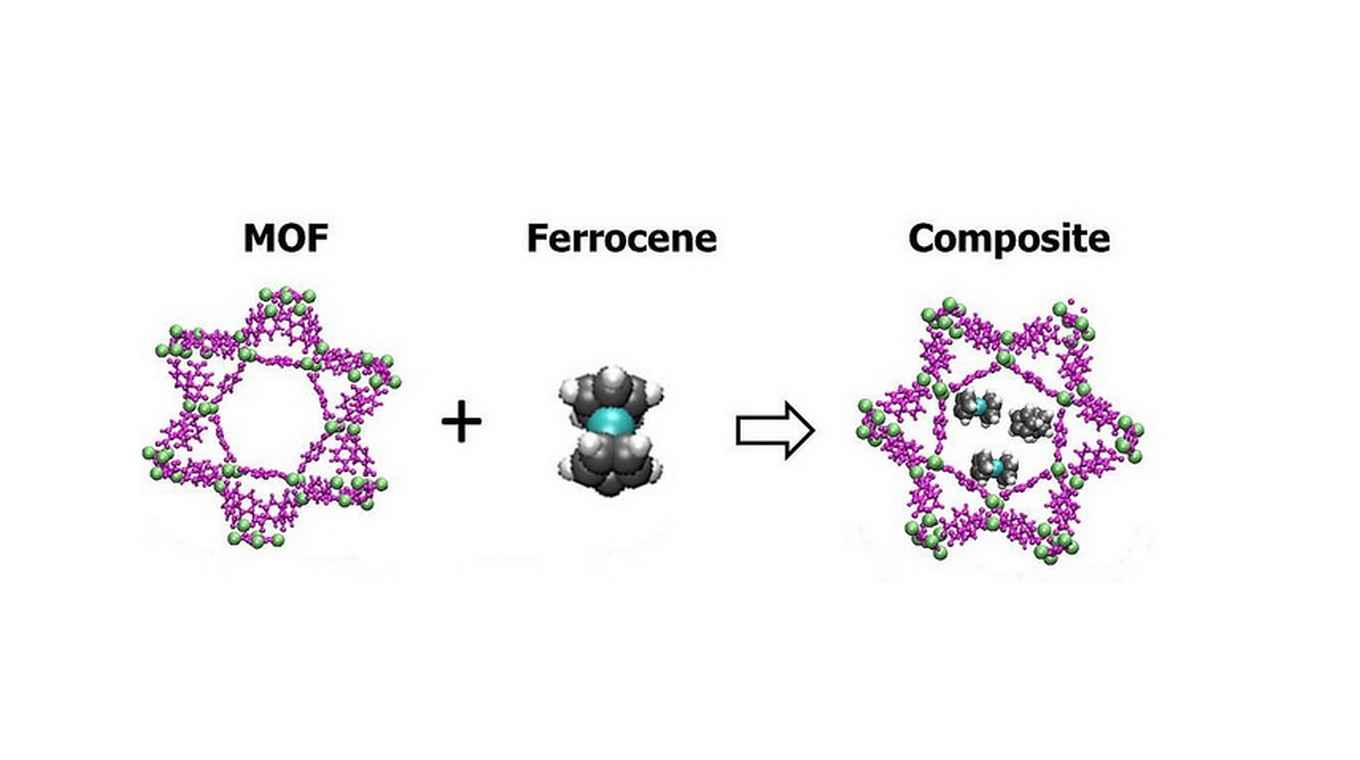
Metal Organic Frameworks are an emerging class of inexpensive materials with great potential for energy efficient separation of gaseous mixtures. MOFs consist of metal ions combined with organic linker molecules to form highly structured porous molecular architectures. The pores can be straightforwardly and rationally tuned to enforce size selective sieving effects. Furthermore the pore surfaces can be readily functionalized to induce preferential interactions with specific molecules.
Professor Krishna of the Computational Chemistry research group at HIMS has performed numerous simulations of the performance of MOFs in a broad range of potential applications. This follows from his continuous focus on improving technologies related to reaction and separation. He is an internationally recognized expert in the investigation of physico-chemical phenomena at the molecular and microscopic levels.
Oxygen separation
In research published online by Advanced Materials earlier this month Krishna cooperated with researchers from Pacific Northwest National Laboratory (PNNL) on the development of an innovative MOF for the simple and cheap separation of oxygen from other gases.
Oxygen separation with MOFs has always been rather problematic since the handful of MOFs known to absorb molecular oxygen also chemically react with the gas, forming oxides. Praveen Thallapally and colleagues at PNNL have now investigated the use of a 'helper' molecule to mediate the oxygen separation. They combined a MOF with ferrocene, an inexpensive iron-containing molecule. Experiments on oxygen mixed with nitrogen, argon and carbon dioxide showed that the MOF/ferrocene composite could indeed be used to separate the oxygen from the other gases.
By using the experimental PNNL data in computer simulations Krishna was able to predict the separation performance of the novel MOF in industrial equipment. Furthermore, comparisons were made with competing materials to establish the practical superiority of the adsorbent developed at PNNL. It has the potential for the development of an easy to prepare, inexpensive and reusable oxygen separator.
Article
Wen Zhang, Debasis Banerjee, Jian Liu, Herbert T. Schaef, Jarrod V. Crum, Carlos A. Fernandez, Ravi K. Kukkadapu, Zimin Nie, Satish K. Nune, Radha K. Motkuri , Karena W. Chapman, M. H. Engelhard, James C. Hayes, Kurt L. Silvers, Rajamani Krishna, B. Peter McGrail, Jun Liu and Praveen K. Thallapally. Redox-Active Metal-Organic Composites for Highly Selective Oxygen Separation Applications Advanced Materials, March 8, 2016, DOI:10.1002/adma.201600259.
Carbon dioxide separation
A second project to which Krishna contributed involved Japanese research into the separation of carbon dioxide from light hydrocarbons. The results, published earlier this month in the print issue of the Journal of the American Chemical Society (JACS), are of relevance for the manufacture of methane via partial oxidation or via hydrocarbon steam cracking of hydrocarbons.
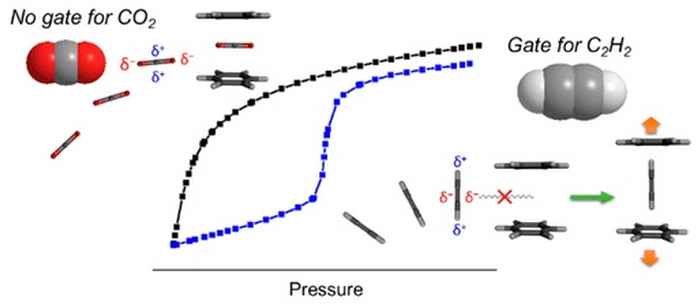
In these processes the removal of carbon dioxide through adsorptive separation is very challenging since the carbon dioxide is very similar to acetylene (C2H2). The two gases have almost the same sorption parameters, physicochemical properties, molecular size and shape.
In JACS the Japanese researchers demonstrate separation of the two gases utilizing the unique structural flexibility of metal− organic frameworks (MOFs) or porous coordination polymers (PCPs) for so-called 'gated adsorption'. There adsorption abruptly increases at a certain gate opening pressure (Pgo) due to a concomitant change in pore structure (induced by structural distortion). The Japanese approach entails the selection of a soft framework that selectively exhibits gate opening for just one of the components, due to differences in electronic properties. The feasibility of this concept was demonstrated with a preliminary gas separation experiment under flowing conditions using a 50/50 CO2/C2H2 gas mixture.
Using the pure component isotherms of CO2 and C2H2 and information on gate opening pressures, Krishna simulated the separation performance in industrial units to demonstrate the practical feasibility of using the novel MOF material.
Article
Maw Lin Foo, Ryotaro Matsuda, Yuh Hijikata, Rajamani Krishna, Hiroshi Sato, Satoshi Horike, Akihiro Hori, Jingui Duan, Yohei Sato, Yoshiki Kubota,Masaki Takata, and Susumu Kitagawa An Adsorbate Discriminatory Gate Effect in a Flexible Porous Coordination Polymer for Selective Adsorption of CO2 over C2H2 J. Am. Chem. Soc., 2016, 138 (9), pp 3022–3030 DOI:10.1021/jacs.5b10491