Molecular 'nickelaburger' shows suprising catalytic activity
9 February 2016

In their Angewandte paper PhD student Daniël Broere, BSc students Eva Blokker and Dieuwertje Modder together with supervisor Dr Jarl Ivar van der Vlugt of the Homogeneous, Bio-inspired and Supramolecular Catalysis research group (part of the UvA Research Priority Area Sustainable Chemistry) describe so-called heterobimetallic compounds with redox-active ligands featuring intramolecular metal-metal interactions. They show that these novel compounds play an essential role in the electrocatalytic activation of carbon-halogen bonds.
Two binding pockets
The new compounds are based on a redox-active PNO ligand previously developed in the Amsterdam research group. This ligand contains two binding pockets, one consisting of a phosphorus atom (P) and the other of an aminophenol group containing nitrogen (N) and oxygen (O) atoms. In earlier studies the redox-active nature of the PNO ligand was used to induce catalytic radical activity for the transition metal palladium (in specific for the intramolecular C-H amination of phenylbutylazide to the N-heterocycle piperidine).
Transition metals are well known for their catalytic activity in a great variety of chemical reactions. There are over 30 of them, all with specific catalytic properties and corresponding applications. Based on their in-depth knowledge of the PNO ligand the researchers decided to investigate its combination with other transition metals. By making use of the difference in chemical “hardness” between phosphorus (P: soft) and the aminophenol pocket (N&O: hard) the researchers were able to selectively bind two metals to the same ligand; each of them to another binding pocket.
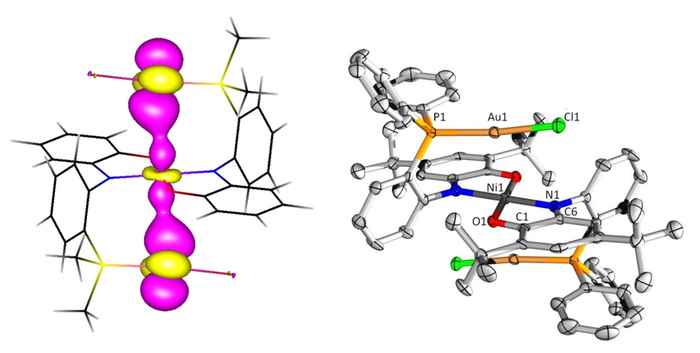
Unexpected catalytic activity
To their surprise the combination of gold (binding to the P pocket) and nickel (binding to the aminophenol pocket) resulted in the formation of an extraordinary Au–Ni–Au ligand complex with a special geometry, more or less resembling a molecular hamburger. They decided to dub it "Aurophilic Nickelaburger with Redox-Active Ligands on the side".
Further research established that their nickelaburger possesses reactivity properties that neither the metals nor the ligand show independently. A striking result was the electrocatalytic C–X bond activation of alkyl halides. Although this was investigated using high-level computational (DFT) calculations, radical trapping experiments and various spectroscopic methods, the mechanistic details remain elusive.
Multi-metallic architectures
It is clear, however, that the presence of a redox-active ligand framework, an available coordination site at gold, and the nature of the nickel–gold interaction are all essential. In future research Van der Vlugt aims to further elucidate why the nickelaburger is unique in displaying the observed reactivity, at the same time expanding his strategy of building complex multi-metallic architectures using redox-active ligands. He expects that other (bioinspired) combinations of multiple metal centers with ligand-based redox-activity will allow novel reaction paths that are not accessible by the individual components.
ERC grant
The research was funded by the European Union through the ERC Starting Grant EuReCat awarded to Jarl Ivar van der Vlugt. The team has collaborated with crystallography expert Maxime A. Siegler at John Hopkins University Baltimore (USA).
Article
Daniël L. J. Broere, Dieuwertje K. Modder, Eva Blokker, Maxime A. Siegler, Jarl Ivar van der Vlugt: Metal-Metal Interactions in Heterobimetallic Complexes with Dinucleating Redox-Active Ligands. Angew. Chem. Int. Ed. 2016, 55, 2406-2410 DOI:10.1002/anie.201509412